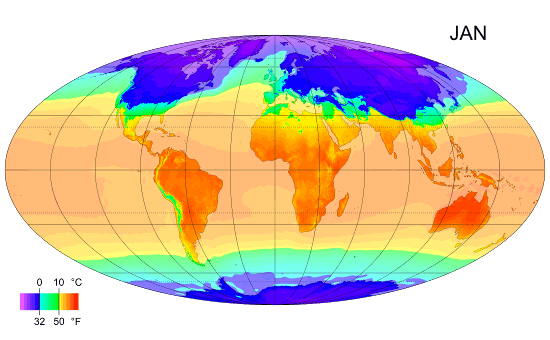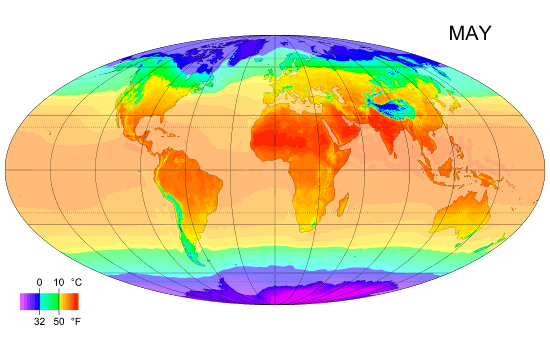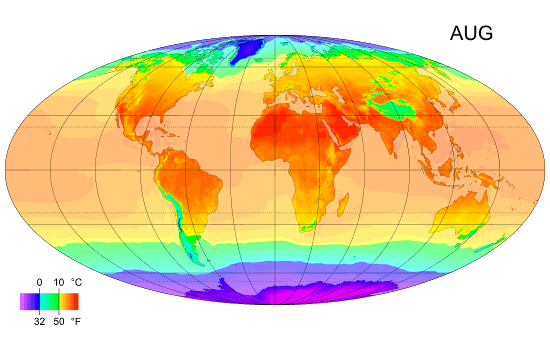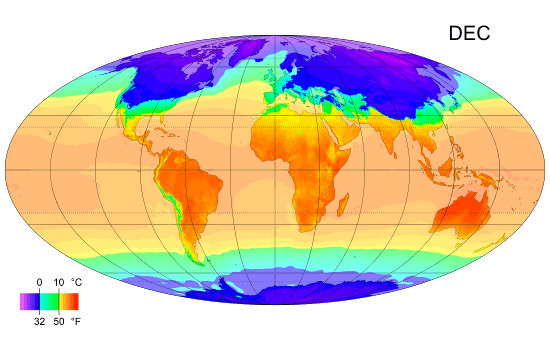
Thermal Physics: Understanding Heat and Temperature
Thermal physics is a branch of physics that deals with the study of heat and temperature. It helps us understand how energy is transferred between different systems and how this affects their temperature. It also helps us understand how temperature influences the behavior of matter, including its phase changes and the way it expands or contracts.
Heat and Temperature
Heat is a form of energy that is transferred from one system to another due to a temperature difference. It is not the same as temperature, which is a measure of the average kinetic energy of the particles in a system. For example, when you touch a hot cup of coffee, heat is transferred from the cup to your hand, causing your hand to feel warm. The cup of coffee has a higher temperature than your hand, so heat flows from the cup to your hand until both reach the same temperature.
Heat Transfer
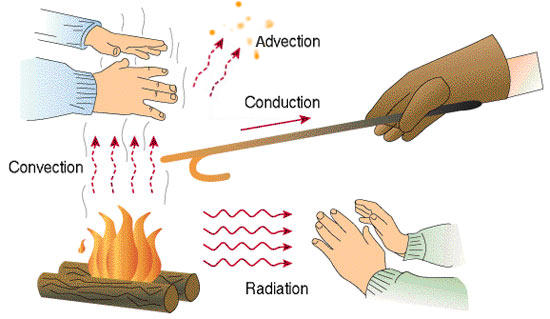
There are three main ways that heat can be transferred: conduction, convection, and radiation. Conduction is the transfer of heat through direct contact between two materials. For example, when you touch a hot stove, heat is transferred from the stove to your hand through conduction. Convection is the transfer of heat through the movement of fluids, such as air or water. For example, when you hold your hand over a candle, heat is transferred to your hand through convection as the hot air rises and cool air sinks.
Radiation is the transfer of heat through electromagnetic waves, such as light or infrared radiation. This can happen even in a vacuum, as heat can be transferred through empty space. For example, when you stand in the sun, heat is transferred to your skin through radiation.
Phase Changes
Temperature also plays a role in the phase changes of matter. When a substance is heated or cooled, it can change from one phase to another, such as from a solid to a liquid or from a liquid to a gas. This is known as a phase change. The temperature at which a substance undergoes a phase change is called its melting point or boiling point, depending on whether it is changing from a solid to a liquid or from a liquid to a gas.
Thermal Expansion
Temperature also affects the way matter expands or contracts. Most substances expand when they are heated and contract when they are cooled. This is known as thermal expansion. For example, when a metal rod is heated, it will become longer as the metal atoms vibrate more and take up more space. This can have practical implications in engineering, as it is important to take thermal expansion into account when designing structures or machines.
Thermal Equilibrium
Thermal equilibrium is a state in which two systems at different temperatures are in contact with each other, but there is no net flow of heat between them. This means that the temperature of the two systems has reached a balance and there is no longer a temperature difference between them. In order for thermal equilibrium to be achieved, the systems must be isolated from their surroundings and any other external influences. When two systems are in thermal equilibrium, they are said to be in thermal contact.
Thermodynamics
Thermodynamics is the branch of physics that deals with the study of heat and its relationship to work and energy. It helps us understand how heat can be transformed into other forms of energy and how energy can be transferred between systems. There are three laws of thermodynamics that describe the fundamental principles of heat and energy. The first law, also known as the law of energy conservation, states that energy cannot be created or destroyed, only converted from one form to another. The second law states that heat can never flow from a colder body to a hotter body without the addition of work. The third law states that as the temperature of a system approaches absolute zero, its entropy approaches a minimum value.
Heat Capacity
Heat capacity is a measure of the amount of heat required to raise the temperature of a substance by a given amount. It is usually measured in units of energy per degree of temperature change, such as joules per degree Celsius. Different substances have different heat capacities, meaning that they require different amounts of heat to raise their temperature by the same amount. For example, water has a high heat capacity, meaning it takes a lot of heat to raise its temperature, while metals have a lower heat capacity and can be heated more easily.
Thermal Conductivity
Thermal conductivity is a measure of how well a substance conducts heat. It is usually measured in watts per meter per degree Celsius. Materials with a high thermal conductivity, such as metals, are good at conducting heat and are often used in heat exchangers and other thermal management systems. Materials with a low thermal conductivity, such as plastics, are poor at conducting heat and are often used as insulation to prevent heat transfer.
Thermal Energy
Thermal energy is the energy associated with the motion and arrangement of atoms and molecules within a system. It is related to temperature, as the higher the temperature of a system, the more thermal energy it contains. Thermal energy can be converted into other forms of energy, such as mechanical energy or electrical energy, through processes such as the expansion of gases or the operation of a heat engine. It can also be transferred between systems through the processes of heat transfer, such as conduction, convection, and radiation.
Conclusion
Thermal physics helps us understand the fundamental principles of heat and temperature and how they affect the behavior of matter. It has practical applications in a wide range of fields, from engineering and materials science to biology and meteorology. By studying thermal physics, we can better understand the world around us and how to harness the power of heat and temperature to our advantage.