Understanding The Laws of Thermodynamics
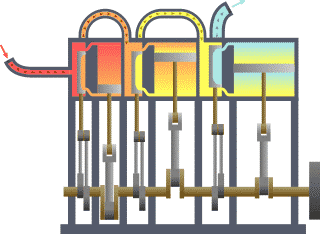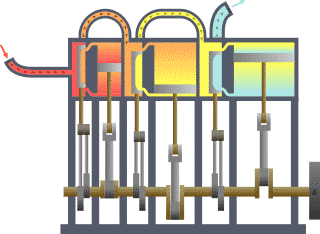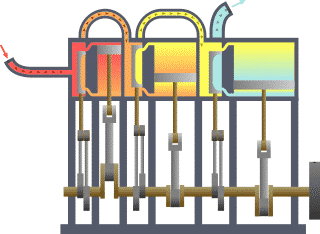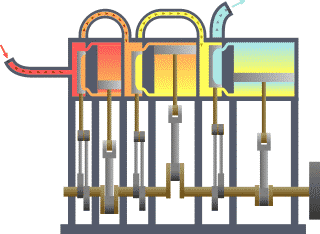
Thermodynamics is the study of the transfer of heat and work in a system. It is a fundamental branch of physics that deals with the behavior of energy and the relationships between heat, work, and energy. There are four laws of thermodynamics that describe the fundamental principles of energy and its relationship to matter. These laws are essential in understanding how energy is used and transformed in various systems.
First Law of Thermodynamics
The first law of thermodynamics, also known as the law of conservation of energy, states that energy cannot be created or destroyed, only transferred or converted from one form to another. This means that the total amount of energy in a closed system remains constant, regardless of the energy transformations that occur within the system. This law can be expressed in the equation:
ΔE = Q - W
where ΔE is the change in energy of the system, Q is the heat transfer into or out of the system, and W is the work done by or on the system.
Second Law of Thermodynamics
The second law of thermodynamics states that the total entropy (a measure of the amount of thermal energy unavailable to do work) of a closed system always increases over time. This means that energy always flows from hotter to cooler systems and that there is a natural tendency for energy to become less useful as it is converted from one form to another. This law is often referred to as the law of increasing entropy or the law of entropy. It can be expressed in the equation:
ΔS ≥ 0
where ΔS is the change in entropy of the system.
Third Law of Thermodynamics
The third law of thermodynamics states that as the temperature of a system approaches absolute zero (the lowest temperature possible), the entropy of the system approaches a minimum value. This means that at absolute zero, all energy is in a state of minimal disorder and the system is in its most stable state. This law is often used to explain why it is impossible to reach absolute zero and why it is so difficult to achieve absolute zero in a laboratory setting. It can be expressed in the equation:
S → 0 as T → 0
where S is the entropy of the system and T is the temperature of the system.
Fourth Law of Thermodynamics
The fourth law of thermodynamics, also known as the law of reversibility, states that it is theoretically possible to reverse the direction of any thermodynamic process, as long as the process is reversible and no energy is lost in the process. However, in practice, it is often very difficult to achieve a completely reversible process due to the inherent loss of energy in most real-world systems. This law is often used in the study of engines and heat pumps to understand the efficiency and limitations of these systems.
In conclusion, the laws of thermodynamics are fundamental principles that describe the behavior of energy and its relationship to matter. These laws are essential in understanding how energy is used and transformed in various systems and have wide-reaching applications in fields such as engineering, physics, and chemistry. For example, the first law of thermodynamics is used to understand the energy efficiency of different devices and systems, while the second law is used to predict the behavior of heat engines and refrigerators. The third law is essential in understanding the behavior of matter at extremely low temperatures, while the fourth law is used to optimize the design of engines and heat pumps. Understanding the laws of thermodynamics is crucial in the development of new technologies and in the optimization of existing systems.
In addition to their practical applications, the laws of thermodynamics also have profound philosophical implications. The second law, for example, suggests that there are limits to the amount of work that can be extracted from a given amount of energy, and that there is a natural tendency for energy to become less useful over time. This has led some scientists and philosophers to consider the concept of a "heat death" of the universe, in which all energy is eventually converted into a low-entropy state and the universe reaches a state of maximum entropy. Despite these philosophical implications, the laws of thermodynamics remain a fundamental and important aspect of modern science and technology.